

Water behaves anomalously below about 30 ☌, at low pressures, increasing pressure reduces viscosity instead of increasing it. As the pressure increases, the volumeĭecreases, and the volume of these voids reduces, so usually Viscous flow occurs by molecules moving through the voids ĭecreases with pressure (at temperatures below 33 ☌) Pr ≪ 1 means thermal conduction dominates over thermal convection (heat diffuses faster than matter moves as with liquid mercury), whereas Pr ≫ 1 means that convection is more effective in transferring energy away from an area, compared to conduction as with motor oils. The Prandtl number is a dimensionless number that compares energy convection and conduction.
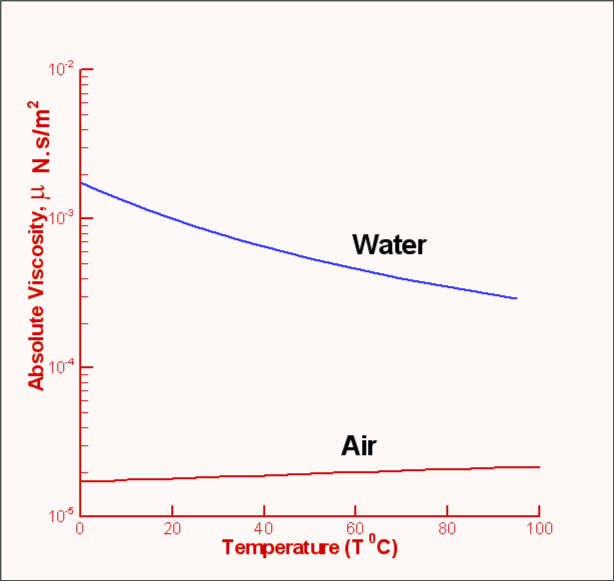
The minimum occurs at about 700 K at 100 MPa (~10 −7 m 2 ˣ s −1). The ArrheniusĮnergy of activation for viscous flow is similar to the hydrogenīond energy (H 2O, 21.5 kJ ˣ mol −1 D 2O,Ģ4.7 kJ ˣ mol −1 T 2O, 26.2 kJ ˣ mol −1,Īt 0 ☌ and all more than doubling at -30 ☌).Īs with other liquids the kinematic viscosity of water has a minimum this is of interest but not an anomaly. Too much difficulty being moved around within organisms. That although the viscosity of water is high, it is not so high that it causes As the hydrogen bonds change with the velocity of flow, the flow-rate affects the viscosity of water. Its extensive three-dimensional hydrogen bonding, and ( at these low temperatures) the viscosity is proportional to the wavenumber of the O-H stretching of water. This cohesiveness is large in water due to At lower temperatures (< 40 ☌) in water, the dominant forces are those holding It depends on the intermolecular forces and molecular momentum exchange. The viscosity of a liquid is the efficiency with which it flows and is determined by the ease with which moleculesĬan move relative to each other. The molar ionic volumes of salts show maxima with respect to temperatureį1 High viscosity (0.89 cP, compare pentane 0.22 cP, at 25 ☌) Some salts prevent the coalescence of small bubbles Some salts give a surface tension-concentration minimum the Jones-Ray effect Of water increases as the density and pressure increase Large diffusion decrease as the temperature is Water's viscosity decreases with pressure 11 (1970).Of the Physical Anomalies of Water (F1-F9) Aleksandrov, Tables of Thermophysical Properties of Water and Water Vapor, Izd-vo Standartov, Moscow (1969).Ī. Golubev, Viscosity of Gases and Gas Mixtures, Fizmatgiz, Moscow (1959). Author's Abstract, Candidates Dissertation, MEI, Moscow (1969).

Levin, The Coefficient of Dynamic Viscosity of Water and Water Vapor. Pustyl'nik, Statistical Methods for Analysis and Processing of Observations, Nauka, Moscow (1968).Ī.


 0 kommentar(er)
0 kommentar(er)
